Don't miss our updates

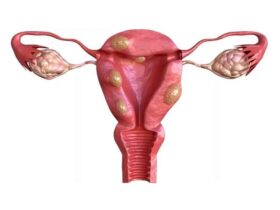
Uterine myomatosis is the formation of myomas or fibroids inside the uterus. These are benign tumors classified according to their location. Depending on where they are found, fibroids are classified as: Intramural: within the muscular wall of the uterus. They can affect fertility if they exceed 4 cm. Submucosal: on the surface of the uterine lining. They hinder embryo implantation. Subserosal: in the outer layer of the uterus. They usually do not interfere with fertility. What is uterine myomatosis and why does it matter for fertility? Uterine myomatosis involves the growth of fibroids, benign smooth muscle tumors, inside the uterus. It affects about 20% of women of reproductive age, especially after age 30. Fibroids can range from microscopic nodules to masses over 4 kg, altering the shape of the uterus and reducing pregnancy chances. How common is uterine myomatosis in those trying to conceive? Uterine fibroids are the most common pelvic tumors in women of reproductive age, present in nearly 70–80% before menopause. Although many are asymptomatic, those that deform the uterine cavity—particularly large intramural and submucosal fibroids—are involved in up to 80% of uterine factor infertility cases. How do fibroids affect embryo implantation? Large submucosal and intramural fibroids can: Alter sperm transport and uterine peristalsis Reduce endometrial receptivity by compressing the lining Increase the risk of implantation failure and early miscarriage Can fibroids complicate pregnancy? Yes. During pregnancy, fibroids can cause: Higher risk of miscarriage and preterm birth Fetal growth restriction due to lack of space Cervical canal obstruction or labor dystocia Postpartum hemorrhage from poor uterine contraction What symptoms may indicate fibroids? About 25% are asymptomatic. When symptoms occur, they include: Sensation of pelvic pressure or heaviness Heavy or prolonged menstrual bleeding Pelvic cramps and irregular bleeding Frequent urination or difficulty urinating Secondary infertility or recurrent miscarriages Severity depends on fibroid size, number, and location. How is diagnosis confirmed? Transvaginal ultrasound is the gold standard, with nearly 100% sensitivity (95% transabdominal). In complex cases, MRI is used. Always consult a reproductive medicine specialist to interpret results and design a treatment plan. Fertility-preserving treatment options Choice depends on age, fibroid characteristics, and reproductive goals: Expectant management for small, asymptomatic fibroids Myomectomy (surgical removal), followed by assisted reproduction techniques Controlled ovarian stimulation combined with in vitro fertilization (IVF) if cavity distortion persists IVF bypasses uterine transport obstacles and allows for selecting the embryo with the highest implantation potential. For more information, see our guide on Endometriosis: what it is and how it impacts fertility. FAQ 1. Can small fibroids disappear on their own? Yes. Many remain stable or shrink after menopause due to estrogen decline. In reproductive age, asymptomatic fibroids under 2 cm are usually monitored with periodic ultrasounds. If you plan to conceive, even a small submucosal fibroid can affect implantation, so consult your doctor to assess intervention before trying to conceive. 2. Are there non-surgical treatments? GnRH agonists and selective progesterone receptor modulators can temporarily shrink fibroids and reduce bleeding in 3–6 month cycles. They improve conditions before surgery or comfort but are not definitive solutions. Never self-medicate without medical supervision. 3. When can I attempt IVF after a myomectomy? Ideally between 6 and 12 months after surgery, before recurrence risk increases. This timing allows the uterine lining to heal and optimizes pregnancy chances. Make sure your specialist confirms proper healing with ultrasound or hysteroscopy before starting treatment. 4. Does the risk of miscarriage increase? Yes. Fibroids that deform the cavity or alter blood flow are associated with early pregnancy loss. Risk increases with fibroid size and location, especially submucosal and large intramural fibroids. Proper management, surgical or with assisted reproduction, significantly reduces miscarriage rates. Always consult a specialist before making decisions. References Faerstein, E., Szklo, M., & Schwingl, P. J. (2001). Risk factors for uterine leiomyoma: a practice-based case–control study. American Journal of Epidemiology, 153(5), 463–469. doi:10.1093/aje/153.5.463 Lau, W., & Shlisselberg, S. (2016). Management of uterine fibroids. American Family Physician, 94(2), 106–113. https://www.aafp.org/afp/2016/0715/p106.html MedlinePlus. (2021). Uterine fibroids. https://medlineplus.gov/uterinefibroids.html Stewart, E. A. (2015). Uterine fibroids. Lancet, 376(9745), 145–157. doi:10.1016/S0140-6736(10)60246-1 Remember: every body is unique. Stay informed, keep hope, and consult an assisted reproduction specialist for personalized care.

Implantation failure is diagnosed when pregnancy is not achieved after at least three cycles of In vitro fertilization (IVF) or after transferring more than ten good-quality embryos. We now have very effective options for every cause of implantation failure: blastocyst-stage transfer (day 5), preimplantation genetic testing, and assisted hatching, among others. Implantation failure: what is it and how does it happen? For an embryo to implant properly, you need a healthy embryo and a receptive endometrium, as well as precise molecular communication. We speak of implantation failure after three IVF cycles without pregnancy or after transferring more than ten embryos with good morphology. Factors that can prevent implantation They are multiple and affect the embryo, the uterus, or the general condition: Embryonic: chromosomal abnormalities, difficulty breaking the zona pellucida, or genetic defects. Uterine: fibroids, synechiae, polyps, infections (hydrosalpinx), scarring, or a thin endometrium (< 7 mm mid-cycle). Systemic: insulin resistance, obesity (BMI > 30), antiphospholipid syndrome, or thrombophilias that alter blood flow. Step-by-step diagnosis To identify the problem, several tests are combined: Blood tests: hormones, metabolic profile, immunological markers, and coagulopathies. Transvaginal ultrasound: uterine malformations, endometrial thickness, and hydrosalpinx detection. Semen analysis and DNA fragmentation: especially if there is a history or male risk factors. PGT-A: preimplantation genetic testing for embryonic aneuploidies. Assisted hatching: study of the zona pellucida in the blastocyst. Reviewing your previous cycles (eggs retrieved, fertilization rate, and embryonic development) is key for an accurate prognosis. Treatment options after repeated failure The strategy will depend on the identified cause: Blastocyst transfer (day 5–6): improves selection of embryos with higher potential. PGT-A: transfer only euploid embryos. Assisted hatching: laser to help the embryo exit the zona pellucida. Hormonal therapies and surgery: for fibroids, polyps, or synechiae. Immunological treatments and anticoagulants: in cases of antiphospholipid syndrome or thrombophilias. Do not self-medicate: always consult a reproductive medicine specialist before starting any drug or treatment. For more details, visit our section on implantation failure or read Implantation Failures: Understanding the Challenge. Frequently Asked Questions 1. Can lifestyle changes improve implantation? Yes. Maintaining a BMI between 18.5 and 24.9, controlling blood glucose, and reducing stress (mindfulness, yoga) favors uterine receptivity and embryo quality. Moderate exercise (30 min/day) and a Mediterranean diet rich in omega-3 balance hormones and reduce inflammation. Quitting smoking and limiting alcohol (< 7 units/week) improves uterine blood flow and decreases oxidative stress. Consult your specialist before making radical changes. 2. How many IVF cycles before diagnosing implantation failure? It is considered failure after three cycles without pregnancy or ten good-quality embryos transferred. However, factors such as age (≥ 38 years), low ovarian reserve, or male factor may prompt earlier evaluation. Your reproductive endocrinologist will guide you on the right timing. 3. How does PGT-A help reduce the risk of miscarriage? PGT-A detects aneuploidies before embryo transfer. Transferring only euploid embryos can reduce miscarriage rates by 50% in women over 35 and increases live births per transfer. Although it raises costs and extends the cycle by about 14 days, it decreases the emotional and financial burden of failed cycles. 4. What immunological treatments are available? In cases of antiphospholipid syndrome or immune alterations, low-dose aspirin (75 mg/day) and heparin (5 000 IU/day) regimens improve uterine perfusion. Some profiles may add IVIG or steroids. An antibody and cytokine panel customizes the therapy; work with a reproductive immunologist to optimize your protocol. Sources Practice Committee of the American Society for Reproductive Medicine. (2018). Evaluation and treatment of recurrent implantation failure: a committee opinion. Fertility and Sterility, 110(5), 704–713. https://doi.org/10.1016/j.fertnstert.2018.08.026 American College of Obstetricians and Gynecologists. (2021). Ovulation induction and assisted reproductive technologies. In ACOG Practice Bulletin No. 208. Obstetrics & Gynecology, 137(1), e49–e64. MedlinePlus. (2023). Embryo transfer. Retrieved from https://medlineplus.gov/embryotransfer.html Ubaldi, F. M., et al. (2017). Culture of human blastocysts with sequential media type: medium and embryo development. Human Reproduction, 32(7), 1424–1432. https://doi.org/10.1093/humrep/dex113 We know how hard this path is. Don’t lose hope: every situation is unique and there are personalized solutions. Consult a reproductive medicine specialist to design the best strategy toward your dream of becoming a parent.

Uterine myomatosis is the appearance of fibroids or fibroids, the most common solid tumors of the uterus in women of reproductive age. They are generally benign estrogen-dependent tumors. They are also called myoma, leiomyoma, or fibroma.Epidemiologically, 2 out of 5 women who present with fibroids do not have any symptoms. They are not a common cause of infertility. For patients who have fertility problems, fibroids have a prevalence of 5-10%. Only in 1 to 2.5% of cases is it a cause of infertility. (1) Among the risk factors that contribute to the development of uterine fibroids are nulliparity, black race, obesity, genetic factors, early menarche, alcohol, and caffeine. (2) The classification of uterine fibroids is based on the relationship they have with the uterine wall, thus they can be subserosal, intramural, and submucosal. 95% of fibroids are located in the body of the uterus, and only 5% are located at the neck. Subserosal fibroids constitute about 10%, originate from the most superficial layers of the uterus, and appear to have no impact on fertility; Intramural fibroids constitute 60 to 70%, they generally do not distort the endometrial cavity, the effect on fertility is not clear or may be minimal when the endometrium is not involved. Submucosal fibroids have a frequency of 15 to 20%, they originate in the myometrium adjacent to the body or cervical uterine mucosa, exerting changes in it, they are the ones that most affect the chances of pregnancy and put an ongoing pregnancy at risk. (3) The symptoms caused by uterine fibroids are related to the location and size of the tumor. In most cases, women with fibroids are asymptomatic. The main symptoms that women report are menstrual disorders, generally with abundant and/or prolonged bleeding that can lead to anemia, pelvic pain, dysmenorrhea, dyspareunia, pelvic heaviness, urinary symptoms, or digestive symptoms. Women with submucosal fibroids more frequently have fertility problems or spontaneous abortions. The explanations are: The diagnosis is usually based on the finding of an enlarged, mobile uterus with irregular contours on physical examination or as an incidental finding on ultrasound. Imaging techniques are useful when it is necessary to confirm the diagnosis or locate the fibroid. Ultrasound is the most widely used diagnostic tool due to its availability and cost/effectiveness. Transvaginal ultrasound has a high sensitivity (95-100%) to detect fibroids in uteruses younger than 10 weeks. The sonohysterogram has greater sensitivity and specificity for submucosal fibroids since it detects the anatomical relationship between the fibroid and the uterine cavity. Magnetic resonance imaging gives better information on the origin of the fibroid. Hysterosalpingography is indicated to study the uterine cavity and the integrity of the uterine ruptures in patients with infertility. If the uterine cavity is normal, there is no advantage in performing a hysteroscopy. If the location of the fibroid is not clear in patients with abnormal uterine bleeding or in those seeking pregnancy, contrast-enhanced ultrasound (sonohysterogram) is the procedure of choice. If imaging studies do not provide an accurate diagnosis, surgical exploration is sometimes required. (5) The treatment of uterine fibroids can be divided into medical and surgical. Medical treatment is associated with inhibition of ovulation, reduction in estrogen production or modification in estrogen and progesterone receptors. Surgical treatment is indicated or recommended in patients with abnormal uterine bleeding that does not respond to medical treatment, high suspicion of malignancy, growth after menopause, infertility with distention of the endometrial cavity or tubal obstruction, pain or a sensation of pressure that interferes with good quality of life, urinary frequency obstruction or disorder, and anemia related to abnormal uterine bleeding. (6) New management presents an alternative to hysterectomy, both safety and effectiveness must be considered in each treatment. It must be recognized that all the new alternatives to hysterectomy allow the possibility of reappearance of undetected leiomyomas mainly because they are small, and may present significant growth, and require new treatment. The risk of recurrence must be balanced with the potential benefits of uterus-sparing procedures, such as decreased morbidity rates and fertility. (7) During the first visit, our patients receive a complete evaluation and adequate classification, mainly in patients with fibroids that involve the endometrial cavity through endovaginal ultrasound, sonohysterogram, and, if necessary, a hysteroscopy. Avoiding at all times unnecessary surgeries that do not contribute to the reproductive goal and/or that put the integrity of our patients at risk. If submucous fibroids < 3cm are present, patients should be managed hysteroscopically. Removing subserosal fibroids is not recommended since they do not contribute to improving the reproductive goal. Patient selection should be individualized based on number, size, and location in addition to the surgeon’s skills. (8) (1)-AAGL Practice Report: Practice Guidelines for the Diagnosis and Management of Submucous Leiomyomas, The Journal of Minimally Invasive Gynecology, 2012(2)- Donnez J, Uterine fibroids management:from the present to the future, Hum Reprod Update, Nov 2016.(3)-SOGC CLINICAL PRACTICE GUIDELINE, The management of uterine fibroids in women with otherwise unexplained infertility, March 2015.(4)-AAGL Practice Report: Practice Guidelines for the Diagnosis and Management of Submucous Leiomyomas, The Journal of Minimally Invasive Gynecology, 2012(5)-E., Pritts, Fibroids and Infertility: an updated systematic review of the evidence, Fertility and Sterility, april 2009.(6)-SOGC CLINICAL PRACTICE GUIDELINE, The management of uterine fibroids in women with otherwise unexplained infertility, March 2015.(7)-SOGC CLINICAL PRACTICE GUIDELINE, The management of uterine fibroids in women with otherwise unexplained infertility, March 2015.(8)-E., Pritts, Fibroids and Infertility: an updated systematic review of the evidence, Fertility and Sterility, april 2009